Sponsored content provided by AstraZeneca
Clinical trials play a critical role in the journey toward medical advancements, bridging the gap between research and real-world applications. However, for many, the process and significance of clinical trials remain daunting and confusing. To simplify this, the below information will help you understand the basics of clinical trials, their importance in developing innovative therapies, and why people of all racial and ethnic backgrounds should consider participating in them.
What are clinical trials?
A clinical trial is a research study performed by doctors and clinical researchers to evaluate the safety of a treatment or better understand a disease. Clinical trials can either be interventional or observational.1
- In interventional clinical trials, researchers assign participants to a specific group. One group receives the treatment being tested, while the other receives an already approved treatment or a placebo (a substance/procedure that looks like a medical treatment but has no therapeutic value). Researchers then compare the results between the groups.2
All medical treatments must be evaluated in an interventional clinical trial before they can be approved by the U.S. Food and Drug Administration (FDA) and made available to the public.3
There are four main phases in interventional clinical trials:3
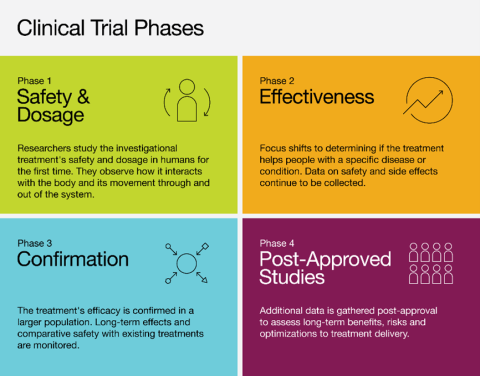
- In observational clinical trials, researchers observe participants during their daily routine or ongoing treatment plan, or review already collected data to track health outcomes over time.2
Are clinical trials safe?
Clinical trials are designed with strict safety measures to ensure participants are protected. This includes:
- Detailed protocols: Before a trial can begin, researchers must develop a protocol—a document that describes the purpose and design of the study, and safety measures that will be taken to minimize participant risk.
- Ethical Review: A panel of reviewers, called the Institutional Review Board (IRB), reviews the protocol to ensure the trial is ethical and minimizes risks.4 The IRB will also monitor the study to make sure steps are being taken to protect the safety of participants.1
- Informed Consent: Participants must consent to joining a trial, showing that they understand the purpose of the study and potential benefits and risks.4
Why are clinical trials important?
Clinical trials are important for determining the benefits and risks of medical treatments. Interventional clinical trials help to gather the data required by the FDA to determine whether a treatment is acceptable for public use; while observational clinical trials help to evaluate the long-term safety, effectiveness and quality of life implications.3
Why is diversity important in clinical trials?
Gender, age, race and ethnicity may impact treatment effectiveness, so it is important that clinical trials reflect the disease community.5
Cardiovascular disease (heart failure, heart disease, stroke, etc.) affects 47% of Black Americans,6 42% of Hispanic Americans,7 and 36% of White Americans.6 Despite the disproportionate impact of the disease on the Black and Hispanic communities, these groups have been underrepresented in cardiovascular clinical trials.8,9
Clinical trial diversity is critical for ensuring the safety and effectiveness of medical treatments. For more information about clinical trials and to learn about clinical trials in your area, click here.
1. Cardiac Amyloidosis: In cardiac amyloidosis, sometimes called stiff heart syndrome, amyloid deposits take the place of normal heart muscle, disrupting the heart's normal structure and function. This can lead to restrictive cardiomyopathy, a condition where the heart becomes stiff and less able to pump blood effectively.5,6
2. Diastolic Dysfunction: Amyloid deposits in the heart can make it difficult for the heart to relax properly during the diastolic phase, impairing its ability to fill with blood. This diastolic dysfunction can result in heart failure with preserved ejection fraction (HFpEF).7
3. Arrhythmias: Amyloidosis can disrupt the heart's electrical system, leading to arrhythmias (irregular heart rhythms) that can further exacerbate heart failure symptoms.7
Diagnosing Amyloidosis
Amyloidosis can be confirmed through specialized tests, including tissue biopsies or imaging scans such as MRIs. Some cases of amyloidosis are hereditary, so if you or anyone else in your family has or had amyloidosis, it can be beneficial for you to take a genetic test to determine if you carry the gene.
Learn More About Amyloidosis
Amyloidosis, which can be a hidden contributor to heart failure, deserves greater recognition and awareness within the medical community and among patients.8 Timely diagnosis and appropriate management can make a significant difference in the prognosis of individuals affected by amyloidosis. 9 For more information on living with amyloidosis, visit MyATTRRoadmap.com.
References
1. National Institute of Health. NIH Clinical Research Trials and You: The Basics https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics [Last accessed 06 28 2024]
2. ClinicalTrials.gov. Study Basics: Learn About Studies. https://clinicaltrials.gov/study-basics/learn-about-studies [Last accessed 06 28 2024]
3. AstraZeneca Pharmaceuticals. What are Clinical Trials? https://www.astrazenecaclinicaltrials.com/what-are-clinical-trials/ [Last accessed 06 28 2024]
4. Healthline. Are Clinical Trials Safe? Your Questions Answered. https://www.healthline.com/health/are-clinical-trials-safe#risks [Last accessed 06 28 2024]
5. U.S. Food & Drug Administration. Clinical Trial Diversity. https://www.fda.gov/consumers/minority-health-and-health-equity/clinical-trial-diversity [Last accessed 06 28 2024]
6. Cleveland Clinic. How Race and Ethnicity Impact Heart Disease. https://my.clevelandclinic.org/health/articles/23051-ethnicity-and-heart-disease [Last accessed 06 28 2024]
7. Gomez, S., Blumer, V., & Rodriguez, F. (2022). Unique Cardiovascular Disease Risk Factors in Hispanic Individuals. Current cardiovascular risk reports, 16(7), 53–61. https://doi.org/10.1007/s12170-022-00692-0
8. Chen, S., & Li, J. (2021). Participation of Black US Residents in Clinical Trials of 24 Cardiovascular Drugs Granted FDA Approval, 2006-2020. JAMA network open, 4(3), e212640. https://doi.org/10.1001/jamanetworkopen.2021.2640
9. U.S. Food & Drug Administration. 2015-2019 Drug Trials Snapshots Summary Report. November 2020. https://www.fda.gov/media/143592/download
Helpful Resources
Visit the HFSA Patient Hub to explore tools and resources to help patients stay healthy while living with heart failure.
View Heart Failure Awareness 365 activities to stay up-to-date on tips for healthy living for people living with heart failure.
