WASHINGTON, DC (SEPTEMBER 10, 2024) - Novel device-based therapies may overcome limitations of pharmacologic therapies for some patients living with heart failure (HF), indicating that a synergistic approach between the two therapies is ideal for implementation of guideline directed medical therapy (GDMT). The Heart Failure Society of America (HFSA) Scientific Statement: Update on Device Based Therapies in Heart Failure, published today in the Journal of Cardiac Failure (JCF), provides a state-of-the-art scientific overview and update of the rapidly evolving field of device-based therapies for HF, including a clinical pathway to implementation of these technologies alongside pharmacologic therapies.
While tremendous advances have been made to reduce hospitalizations and improve outcomes for patients with HF in the past decade, the residual risk on optimized guideline directed medical therapy (GDMT) remains on par or worse than other major cardiovascular diseases. Some established medical devices, such as implantable cardioverter-defibrillator (ICD) and cardiac resynchronization therapy (CRT), are widely used alongside pharmacologic therapies. However, in some high-risk patients that exhibit an intolerance for certain drugs, novel device therapies may prove highly effective in producing promising outcomes.
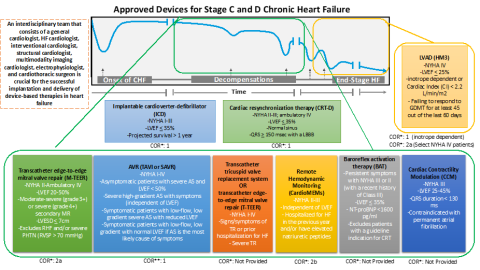
The Update on Device Based Therapies in Heart Failure scientific statement defines how these novel device therapies may bridge current gaps in HF treatment and outcomes and proposes a clinical pathway to implement FDA approved device-based therapies that align with current HF management workflow.
" Our consensus document defines the unmet need in chronic heart failure and the important role of device-based therapies in bridging heart failure gaps,” said co-lead author Jerry D. Estep, M.D., FACC, FASE (Division Chair, Cardiovascular Medicine, Cleveland Clinic Florida). "We provide detailed information on the different categories of heart failure devices and use considerations based on associated contemporary outcome data. We hope our document will improve patient outcomes by providing a clinical framework to guide implementation of current and future FDA approved device-based therapies."
Novel FDA-approved devices for consideration in the statement include cardiac contractility modulation (CCM), baroreflex activation therapy (BAT), valve interventions with transcatheter aortic valve replacement, mitral valve edge-to-edge repair, tricuspid repair, CardioMEMS pulmonary artery pressure monitoring, and HeartMate [HM] 3 left ventricular assist device [LVAD].
The Update on Device Based Therapies in Heart Failure scientific statement proposes a clinical pathway to implementation that involves the consideration of device-based therapies following a persistence of NYHA class II or above symptoms after 3-6 months of pharmacological GDMT and CRT. It also suggests that there must be improved systems of evaluating appropriate patient populations for these therapies, as they may be underutilized, especially in cases where patients may be eligible for more than one device-based therapy. Overall, the scientific statement suggests that a personalized approach to selecting therapies when implementing GDMT will most benefit patient outcomes.
The Heart Failure Society of America (HFSA) Scientific Statement: Update on Device Based Therapies in Heart Failure is available online in the JCF at www.onlinejcf.com.
DOI: https://doi.org/10.1016/j.cardfail.2024.07.007